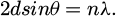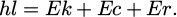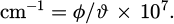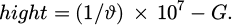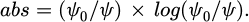| Issue |
Manufacturing Rev.
Volume 12, 2025
|
|
|---|---|---|
| Article Number | 17 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/mfreview/2025012 | |
| Published online | 18 July 2025 | |
Original Article
Preparation and performance of modified MWCNTS nanocomposites for ship anti corrosion
College of Marine and Electrical Engineering, Jiangsu Maritime Institute, Nanjing, 211100, China
* e-mail: lzljmi@126.com
Received:
2
January
2025
Accepted:
16
June
2025
To improve the anti-corrosion and waterproof performance of marine vessel surfaces, many acrylic resin coating materials have been proposed, but many of them still have the problem of low anti-corrosion performance. To address this problem, nanocomposites based on modified multi-walled carbon nanotubes are proposed in the study with a view to improving the anticorrosive properties of acrylic resin coating materials. The study first examined the structure of the nanocomposite using relevant instruments. The results showed that the nanocomposite was able to mix SiO2, multi-walled carbon nanotubes, and 1H,1H,2H,2H-perfluorodecyltrimethoxysilane efficiently with good waterproofing and anticorrosion properties. Subsequently, the nanocomposites were applied to acrylic resin coating materials for experiments. The outcomes revealed that the coating material had a contact angle of 131.8° and an average impact strength of 58.3 kg·cm. The impedance value was much higher than 109 Ω·cm2 after immersion in 3.5 wt% NaCl solution for 8 days, and the impedance value was stable. The above results indicate that the nanocomposites based on modified multi-walled carbon nanotubes proposed in the study can improve the anticorrosive properties of acrylic resin coating materials. This study provides a scientific basis for improving the anticorrosion performance of marine vessel surfaces.
Key words: Multi-walled carbon nanotubes / acrylic resin / ship / coating / corrosion prevention
© J. Hui and C. Sun, Published by EDP Sciences 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
With the continuous development of modern marine industry, corrosion of materials by marine environment has become a major challenge for marine ship transportation [1]. The high salinity, high humidity and complex microbial environment of the marine environment exposes the ship's surface materials to severe corrosion risk, which seriously affects the ship's service life and safety. Facing this challenge, many scholars have proposed the use of acrylic resin (AR) coating materials to protect ship surfaces [2]. However, due to the problem of small holes in the curing process of AR material, the corrosion resistance (CR) of the ship surface is often reduced. In view of this, it is particularly urgent to develop a modification method that can effectively improve the anti-corrosion properties of AR materials. At present, the modification of AR by introducing other composite materials has proved to be a scientific and effective method. Among which multi-walled carbon nanotubes (MWCTNs) are widely used due to their advantages such as excellent thermal conductivity (TC) and barrier properties [3]. Nanocomposites by adding MWCTNs exhibit excellent properties such as good barrier and CR [4]. Many scholars have studied them. For example, to investigate the effect of MWCNTs on polypropylene nanocomposites, JSabet conducted experiments using twin-screw extruder and injection molding techniques. The experimental results showed that MWCNTs significantly increased the thermal stability of polypropylene nanocomposites [5]. In order to improve the physical properties of epoxy resin/carpet waste polymer composites, Kumar et al. proposed to add MWCNTs to this composite. Through the experiments, the results showed that MWCNTs increased the flame retardancy and compression resistance of this composite [6]. To address the problem of electromagnetic pollution in electromagnetic wave absorbers, Jia et al. proposed a method to prepare Co/ZnO/C@MWCNTs composites by pyrolyzing ZnCo-MOF@MWCNTs. The experimental results indicated that changing the content of MWCNTs in the composites could improve the impedance matching performance of the materials [7]. To address the issue of steel structures' poor CR, Rezaei Abadchi M. et al. proposed an AA layer containing titanium oxide, zinc phosphate, and benzophenone. Experiments showed that this material had good anti-corrosion performance [8]. To address the shortcomings of metal surface coatings, such as poor heat and CR, Jiang W. et al. proposed a material that directly blended and modified organosilicon-AA with graphene oxide's two-dimensional flake structure. Experimental results showed that the modified coating material exhibited improved CR [9].
However, MWCTNs suffer from defects such as poor dispersion and susceptibility to agglomeration [10]. Silicon dioxide (SiO2) nanoparticles are inorganic chemical materials that can compensate for some of the defects of MWCTNs by having advantages such as good chemical stability and improved stability and thixotropy of coating materials [11]. Nevertheless, the composites prepared by combining MWCTNs and SiO2, applied to enhance the performance of AR coating materials, still suffer from poor CR and water resistance [12]. 1H,1H,2H,2H-perfluorodecyltrimethoxysilane (FAS) has the advantages of low surface energy and very poor wettability, which is resistant to hydrolysis, and is widely used in the fields of materials and energy [13]. The above results show that although the current modification methods of MWCTNs have some effect, they still cannot solve the problems of easy aggregation and poor dispersion of MWCTNs. Therefore, in this study, nanocomposites (MWCTNs-OH@SiO2) are prepared by combining SiO2 and MWCTNs with the introduction of hydroxyl (−OH). Subsequently, FAS is introduced to modify them and finally modified MWCTNs composite nanomaterials are obtained. The innovation of this research is to prepare a nanocomposite prepared material by combining SiO2, MWCTNs, and −OH. It is also modified using FAS to improve the properties of AR coating materials, such as anti-corrosion, waterproofing, and hydrophobicity. This modification also improves the corrosion and fouling resistance of marine vessel surfaces.
2 Materials and methods
2.1 Preparation of modified MWCNTs nanocomposites
2.1.1 Characterization and current status of MWCNTS applications
The complex conditions of the marine environment, such as high salt content and humidity, lead to severe corrosion of the ship's surface. Therefore, improving the anti-corrosion performance of the ship's surface is an urgent problem that must be solved. AR material is widely used in ship surface anticorrosion, but its anticorrosion performance is still insufficient. To solve this problem, a modification scheme based on nanocomposite material is designed to improve the anti-corrosion performance of AR material. The nanocomposite is composed of SiO2, MWCNTs, -OH and FAS. Before preparing modified MWCNTs composites, it is necessary to have a comprehensive understanding of their properties and application status. MWCTNs are carbon nanomaterials with a seamless nanotube-like structure consisting of multilayered graphene sheets convoluted [14]. The material has several excellent properties including mechanical, electrical, and thermal properties. Among them, the mechanical properties are characterized by a tensile strength 100 times stronger than that of steel and a density of only 1/6 of that of steel, as well as excellent elasticity and fast recovery [15]. The electrical properties are characterized by excellent electrical conductivity, which far exceeds that of copper [16]. The thermal properties are characterized by an axial TC comparable to that of metals and much higher than that of many conventional materials [17]. These characteristics give the material a broad range of uses in flexible electronics, electronic components, and structural materials. Figure 1 depicts the MWCTNs' construction [18].
In Figure 1, the MWCTNs are in a layered structure, with each carbon atom in each layer connected to three neighboring carbon atoms to form a hexagonal lattice structure. The structure is formed because the carbon atoms in MWCTNs are predominantly sp2 hybridized. This structural feature makes MWCTNs have physical and chemical properties such as high strength and high TC. In the field of ship anticorrosion, this material is used to enhance the anticorrosion performance of waterborne acrylic coating materials and improve the hydrophobicity of the coating surface through molecular design and copolymerization. In other fields, MWCTNs can be added to composite materials to improve TC and CR. In summary, MWCTNs have the ability to enhance the TC and CR of materials, and have great potential for application. Although there are more methods of modifying MWCTNs applied to AR coating materials for ships, there is still the problem of poor CR. In contrast, the anticorrosive qualities (AP) of the coating can be enhanced by using SiO2 to cover the small holes and cavities in the film-forming material of the coating. This will prevent the corrosive electrolyte between the coating and the ship's surface. SiO2 and MWCTNs together can maximize their complementing benefits and improve coating AP. Modification of the composites of MWCTNs and SiO2 using -OH can improve the compatibility of the inorganic filler in AR, thus enhancing the AP of the coating materials. The use of FAS can convert the hydrophilicity of the material into water repellency and increase the isolation ability of the inorganic filler from corrosive media, thus improving the anti-corrosion property of the coating. At present, the analysis techniques for modified MWCNTs are X-ray diffraction analysis and infrared spectroscopy. Among them, the principle of X-RDA is based on the diffraction effect of X-rays to analyze the crystal structure of substances, which mainly uses the Bragg equation. The calculation formula is shown in equation (1).
Equation (1) is the d interval between crystallographic surfaces. Θ is the diffraction angle. λ is the wavelength. n is an integer. The basic idea behind X-ray photoelectron spectroscopy is that when a sample or material is exposed to a certain amount of X-ray radiation at its surface, the electrons in the atoms of the material can separate and become free electrons. Equation (2) can be used to express this process.
In equation (2), hl is the energy of the X-photon. Ek is the energy of the photoelectron. Ec is the binding energy of the electron. Er is the recoil energy of the atom. The underlying principle of infrared spectroscopy entails the utilization of molecules' absorption properties for infrared rays of varying wavelengths, as depicted by the wave number calculation expression presented in equation (3).
In equation (3) cm−1is the wave number. ϕ is the speed of light. ϑ is the wavelength. The expression for the calculation of the absorption peak hight is shown in equation (4).
In equation (4), G is a fixed constant. The expression for the calculation of absorption intensity abs is shown in equation (5).
In equation (5), ψ0 and ψ are the initial light intensity and the light intensity through the sample, respectively.
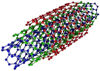 |
Fig. 1 Schematic diagram of MWCTNS. |
2.1.2 Preparation of modified MWCNTs nanocomposites
After an in-depth study of the characteristics and application status of MWCNTs, it is learned that MWCNTs have been widely used in many fields due to their unique structural characteristics and excellent mechanical properties. Due to their exceptional mechanical properties and unique structural characteristics, MWCNTs have found extensive application in a variety of industries. The study uses SiO2, -OH, and FAS to modify MWCNTs. The preparation method is shown in Figure 2.
In Figure 2, the preparation method of modified MWCNTs is divided into three steps. One is the preparation of MWCNTs-OH, the other is the preparation of MWCNTs-OH@SiO2, and the third is the preparation of FAS-MWCNTs-OH@SiO2. Among them, MWCNTs-OH is prepared by slowly injecting ultrapure water into a beaker containing MWCNTs-OH and stirring to decompose MWCNTs-OH and then dispersing it using an ultrasonic cleaner. After dispersion, the suspension of MWCNTs-OH is contained in a three-mouth flask. After that, a rubber-capped buret is employed to slowly drop a mixture of tetraethyl silicate (TEOS), deionized water (H2O), and CH3CH2OH into the beaker in the ratio of 3:1:4, which totals 20 mL. A blender is employed for mixing in the process, and after the mixing is completed, an ultrasonic cleaner is employed for dispersing the solution for 2 h. This results in a MWCNTs-OH suspension. Next, SiO2 is added to the beaker and stirred by a magnetic stirrer, after which it is placed at room temperature. After 24 h, it is filtered using a high-speed centrifuge. After the filtration is completed, CH3CH2OH is used to clean it for three times, and it is filtered after being cleaned off. Moreover, a vacuum drying oven is used to dry the treatment and then grind it, thus obtaining MWCNTs-OH@SiO2. Finally, after weighing 0.5 g of MWCNTs-OH@SiO2 powder on a balance, it is added to 100 mL of anhydrous CH3CH2OH in a conical flask. Subsequently, stirring is carried out, and after stirring is completed, the obtained mixed solution is ultrasonically dispersed using an ultrasonic cleaner. Following 30 min of ultrasonic dispersion, the mixture is supplemented with 2 ml of ultrapure water, acetic acid, and a suitable quantity of FAS. After the addition is completed, an oil bath is used for the reaction. After 4h of reaction, a high-speed centrifuge is used for filtration. After filtration, it is placed in the environment of 80 °C for dehydration, thus obtaining FAS-MWCNTs-OH@SiO2. After obtaining it, it is ground and then encapsulated for storage.
 |
Fig. 2 Preparation method of modified MWCNTs. |
2.1.3 Modified MWCNTs nanocomposites preparation apparatus and reagents
In the process of modifying MWCNTs, some experimental instruments and reagents are required. For the preparation of modified MWCNTs nanocomposites, the reagents and apparatus used for the experiment are shown in Table 1.
The reagent specifications used in Table 1 are all analytically pure. Scanning electron microscope and projection electron microscope from Zeiss (Germany) and Thermo Fisher (Netherlands) are also used.
Experimental instruments and reagents.
2.2 Modified MWCNTs nanocomposites in modified AR
2.2.1 Preparation of modified AR
To better improve the anti-corrosion properties of AR materials, AR is modified after understanding the preparation method of modified MWCNTs. To solve the problem of corrosion of ships by the marine environment, many scholars have proposed to modify MWCNTs and add them into AR coatings to improve the anticorrosion performance of ship surface coatings [19]. However, AR has the defects of poor thermal stability and weak antifouling performance. Therefore, it is necessary to modify AR before applying it with the modified MWCNTs prepared in the study.
In Figure 3, the modification process is firstly to add 125 mL of sodium hydroxide to a beaker containing 20 mL of zinc acetate of 3.5 mol/L concentration using a dropper. After it is fully reacted, it is filtered, washed and dried to obtain alkaline zinc acetate. Next, it is ground into powder form, 71 g of n-butanol is added, stirred for 30 min and then packed into a three-necked flask (3-NF) and put into an oil bath having a temperature of 70°C. Next, 14.4 g of acrylic acid and 20 g of n-butanol are added to a beaker. It is stirred well and then dripped into a 3-NF using a constant pressure dropping funnel and stirred for 3h to 4h after dropping. After that, the water is removed using a rotary evaporator to obtain a solution of zinc acrylate monomer. Immediately after that, N-phenylmaleimide is employed by weighing 22.36 g of N-phenylmaleimide, grinding it into powder form and placing it into a beaker containing 60 mL of N-dimethylformamide for ultrasonic stirring. Then, 9 g, 37.5 g, and 28.5 g of zinc acrylate, butyl acrylate, and methyl methacrylate are taken into the beaker containing the solution, respectively. At the same time, 2.25g, 10g, and 10g of azobisisobutyronitrile, xylene, and n-butanol are weighed into a beaker for stirring and then poured into a 3-NF. Finally, 0.75g, 20g, and 17g of azodiisobutyronitrile, xylene, and n-butanol are weighed into a 100 mL beaker and then put into a 3-NF using a constant pressure dropping funnel. It is stored at a temperature of 85°C. After 4h, it is dried by rotary evaporator to remove the organic solvent. After 24h, the modified AR is obtained. The study is verified by infrared spectroscopy, nuclear magnetic resonance hydrogen spectroscopy, thermal analysis, and gel chromatography that the modified AR has good stability, film-forming, and other properties.
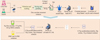 |
Fig. 3 Preparation process of modified AR. |
2.2.2 modified Experimental drugs and apparatus for MWCNTs and AR composites
In conducting the experiments on composites based on modified MWCNTs, the first step is to prepare the composite coatings (CCs). The sources of drugs and reagents needed are shown in Table 2.
The reagents used in Table 2 are test grade. In addition to this an electrically heated constant temperature incubator from Shanghai Zhicheng and a scanning electron microscope from Zeiss, Germany are also used.
Sources of experimental drugs and reagents.
2.2.3 Application of modified MWCNTs in modified AR and experimental methodology
After the modification of AR, the study applies modified MWCNTs to it to prepare composite coating material (CCM) based on modified MWCNTs, and conducts performance test experiments on it after successful preparation. The preparation process of CCM based on modified MWCNTs is shown in Figure 4.
In Figure 4, the preparation process of this CC firstly adopts a beaker to mix 0.5L of dibenzyl and n-butanol and keep it. Second, 0.2g of FAS-MWCNTs-OH@SiO2 powder is weighed into it and dispersed for 30 min using ultrasonic cleaner. Next, 20g of modified AR is weighed into a beaker using a balance and then placed on a magnetic stirrer, while the mixed solution obtained in the previous step is slowly poured into the beaker containing the modified AR and stirred using a magnetic stirrer. After 12h, it is ball milled using a ball mill. After 6h, diphenylmethyl is added and then it is stirred into filaments using a paint mixing knife. Finally, it is stored in a vacuum environment until there are no air bubbles.
In the experiments, the CCM based on modified MWCNTs is sprayed on the substrate made of mild steel and tinplate by using a spray gun using the hybrid spraying method. Finally, the CR of the CCM based on modified MWCNTs is tested by electrochemical impedance spectroscopy.
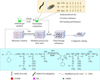 |
Fig. 4 Preparation process of CC materials for modified MWCNTs. |
3 Results
3.1 Analysis of preparation results of modified MWCNTs nanocomposites for ship anticorrosion
After the preparation of modified MWCNTs, the properties of MWCNTS are analyzed. In this experiment, the modified MWCNTs are prepared with 0.5g MWCNT-OH, 20 ml TEOS, 20 ml H2O, 100 ml ultrapure water, 100 ml anhydrous CH3CH2OH, 0.5gWCNTs-OH@SiO2, and 2 ml acetic acid. To examine the results of the preparation of modified MWCNTs nanocomposites, the study uses X-RDA and infrared spectrum analysis to examine the effect of FAS on the structure of MWCNTs-OH and MWCNTs-OH@SiO2. Figure 5 displays the findings.
In Figure 5a, the diffraction spectrum curves of MWCNTs-OH, MWCNTs-OH@SiO2, and FAS-MWCNTs-OH@SiO2 all exhibit three obvious diffraction peaks. The intensity and position of the diffraction peaks of MWCNTs before and after modification show very little change. Therefore, it can be explained that the modification of MWCNTs did not change their overall chemical structure. The results show that the modified MWCNTs maintain their original properties and combine the properties of SiO2 and FAS, and the modification is effective. In Figure 5b, the IR spectra of modified MWCNTs exhibit stretching and vibration phenomena, and these belong to the absorption peaks of MWCNTs-OH and MWCNTs-OH@SiO2, indicating that SiO2 and FAS are absorbed. The results prove that SiO2 and FAS are encapsulated on MWCNTs, and FAS and SiO2 are successfully grafted on MWCNTs. The X-ray photoelectron spectroscopy analysis results of the modified MWCNTs are shown in Figure 6.
In Figure 6a, the MWCNTs-OH@SiO2 composites that are not modified by FA contain elemental peaks with C, Si, and O elements. When modified by FAS, one more higher elemental peak is F. This result indicates that the introduction of FAS makes the characterization of MWCNTs-OH@SiO2 exist with F element. In Figures 6b, 6c, and 6d, several elemental peaks of -OH, O-Si, Si-C, and Si-O appear after fitting. The O-Si chemical bond originates from the SiO2 that encapsulates the MWCNTs. The Si-C originates from the FAS. The Si-O comes from the dehydration synthesis reaction that occurs between the FAS and the MWCNTs-OH@SiO2. The above results indicate that the dehydration reaction of FAS with MWCNTs-OH@SiO2 formed Si-O chemical bonding, which enables FAS to successfully access the surface of MWCNTs-OH@SiO2. To further examine the performance of the modified MWCNTs proposed in the study, the microscopic morphology of MWCNTs-OH, MWCNTs-OH@SiO2-OH, and FAS-MWCNTs-OH@SiO2 is investigated and analyzed by scanning electron microscopy. The analyzed results are shown in Figure 7.
In Figure 7a, the surfaces of MWCNTs-OH are chaotic and intertwined with each other, forming an intertwined thread-like structure. In Figure 7b, the surface of MWCNTs-OH@SiO2-OH can be visualized to be scattered with fine particles, which indicates that SiO2 successfully covers the MWCNTs. In Figure 7c, the tightness of the FAS-MWCNTs-OH@SiO2 surface interlacing is reduced, a looser structure is formed, and a smoother film is formed on the surface. This further confirms that FAS has been successfully modified on the surface of MWCNTs-OH@SiO2, thus enhancing its water repellency and anticorrosion properties. The outcomes display that the MWCNTs are modified with good results and have good water repellency and AP, and the performance is better than that before modification. In Figure 7, MWCNTs-OH is 0.5g, without FAS and SiO2, MWCNTs-OH@SiO2 is MWCNTs-OH with 0.2g SiO2 and 0.5g, FAS-MWCNTs-OH@SiO2 0.2g SiO2, FAS and 0.5g MWCNT-OH are added, respectively. In addition, in order to verify that the chemical structure of MWCNTs do not change with the addition of SiO2 and FAS, thermogravimetric analysis is performed on them. The analysis results are shown in Table 3.
In Table 3, the chemical structure of MWCNTs do not change with the addition of FAS and SiO2. The weight loss occurs mainly in the specific temperature range, which is related to the physical adsorption and grafting of the filler rather than the chemical changes of the MWCNTs themselves.
 |
Fig. 5 X-ray diffraction analysis and infrared spectrum analysis results of MWCNTs-OH and MWCNTs-OH@SiO2. |
 |
Fig. 6 X-ray photoelectron spectroscopy of modified MWCNTs. |
 |
Fig. 7 Micromorphologies of MWCNTs, MWCNTs-OH@SiO2, and FAS-MWCNTs-OH@SiO2. |
Analysis results.
3.2 Analysis of application results of modified MWCNTs nanocomposites
After verifying the modification results and waterproof performance of modified MWCNTs composites, the study conducts contact angle (CA) test, adhesion test, impact resistance test, flexibility test, and anticorrosion performance of CC based on modified MWCNTs. Among the components of the CC, the modified MWCNTs are 0.5g, the modified AR is 20g, and the xylene and n-CH3CH2OH are 5 ml. The results of these tests are shown in Figure 8.
In Figure 8, A is AR and F is modified MWCNTs (FAS-MWCNTs-OH@SiO2). In Figure 8a, with the addition of FAS-MWCNTs-OH@SiO2 in modified AR from 1 wt%–3 wt%, its CA is enhanced from 97.7° to 131.8°. Relative to the modified AR without the addition of modified MWCNTs, its CA increases by 64.74°. The larger the CA, the stronger the water repellency. The results suggest that the water repellency of the CC is optimal at 3 wt% FAS-MWCNTs-OH@SiO2 addition. However, the water CA of the CC becomes smaller at 4 wt% of modified MWCNTs addition. It indicates that the excess filler causes the accumulation of film-forming resin, resulting in a smaller CA. In Figure 8b, the adhesion of the CCs is at level 1 for the addition of modified MWCNTs at 0 wt%–3 wt% and at level 2 for the addition of 4 wt%. The adhesion of the CCs has a total of 10 levels, which decreases as the number increases. This result shows that the coating has good adhesion. In Figure 8c, the average impact strengths of the three CC samples are 38.3 kg·cm, 48.3 kg·cm, 53.3 kg·cm, 58.3 kg·cm, 48.3 kg·cm, respectively, for modified AR with the addition of 0 wt%–4 wt% modified MWCNTs. Among them, the impact resistance of the CC gradually increases at 0 wt%–3 wt% additions. It decreases at 4 wt% addition because excessive addition of FAS-MWCNTs-OH@SiO2 leads to the appearance of severe agglomeration phenomenon, which increases the number of pores in the CC and decreases the impact resistance. In Figure 8d, the flexibility of the coatings is optimized when the quantity of FAS-MWCNTs-OH@SiO2 added in modified AR is 2 wt% and 3 wt%. The above outcomes show that the CCs have the optimal impact resistance, water resistance, flexibility, and adhesion when the amount of FAS-MWCNTs-OH@SiO2 is added to the modified AR at 3 wt%. To further examine the performance of modified MWCNTs CC, it is tested for CR. The test method is electrochemical resistance spectroscopy, and a three-stage electrical system and ZSimp Win software are used to fit the CR parameters of the modified MWCNTs CC. The results of Nyquist curve and Bode map of the CC immersed in 3 wt% NaCl solution (NaCl-S) for 8 days are shown in Figure 9.
In Figure 9, the resistance values of the CCs of 1 wt%–4 wt% FAS-MWCNTs-OH@SiO2 are much higher than 109 Ω·cm2 after immersed in 3.5 wt% NaCl-S for 8 days. Furthermore, the resistance values of all CCs are in a steady state with slow fluctuations. This result shows that the CC with 1 wt%–4 wt% FAS-MWCNTs-OH@SiO2 additions still has a strong CR at the immersion time of 8 days. Table 4 displays the findings of the FAS-MWCNTs-OH@SiO2 CC self-healing ability (SHA) test.
In Table 4, Zw is the Warburg impedance, Qc is the constant phase angle element (CPAE) of the coating, Qd is a double-layer capacitor, Rct is the charge transfer resistance of the metal, and Qd is the CPAE of the capacitor. The dn and cn are the CPAE of the coating and the CPAE index of the capacitor, respectively. At the immersion time of 6 h, the metal substrate at the scratch is easily attacked by the corrosive medium. At this time, the Rct is 1.107 × 104 Ω·cm2. When the immersion time is 30h, the Rct of the coating is 1.025 × 106, which is 100 times the value of the Rct of the coating at the immersion time of 6h. This result shows that the CC has excellent SHA and anti-corrosion ability.
 |
Fig. 8 Results of contact angle test, adhesion test, impact resistance test, and flexibility test. |
 |
Fig. 9 Nyquist curve and Bode map results of CC immersed in 3 wt%NaCl-S for 8 days. |
Test results of SHA of FAS-MWCNTs-OH@SiO2 CC.
4 Discussion
To test the properties of modified MWCNTs nanocomposites, the study was carried out to test the properties of modified MWCNTs by using X-RDA, infrared spectrum analysis and performance testing experiments using swept electron microscopy. The results of X-RDA revealed that the intensity and position of diffraction peaks of MWCNTs changed very little before and after modification. It indicated that the modification of MWCNTs by SiO2 and FAS did not change the overall chemical structure of MWCNTs, and retained the original properties while possessing the advantages of SiO2 and FAS, and the modification was effective. This experimental result was similar to that of Liu et al. This was due to the fact that the incorporation of FAS and SiO2 mainly enhanced the properties of the composites through physical encapsulation, maintaining structural stability, synergistic effects as well as protective and dispersive effects [20]. The presence of F elements in the surface features of modified MWCNTs was demonstrated by the experimental results of the infrared spectrum analysis. This result indicated that FAS successfully accessed MWCNTs-OH@SiO2. This experimental result coincided with that of Meng et al [21]. In addition, in the swept electron microscopy experimental test experiments, the results indicated that the surface of modified MWCNTs possessed a film with a loose and smooth structure. Its waterproof and AP were better than those of MWCNTs and MWCNTs-OH@SiO2. The study also conducted experimental tests on CA test, impact resistance test, flexibility, AP, and SHA of the CCM based on modified MWCNTs. In the CA test, adhesion test, impact resistance test, and flexibility experimental test, the coating material had a CA of 131.8° and an impact strength of 48.3 kg·cm when the amount of added MWCNTs was 3 wt%, which were superior to the coatings without added modified MWCNTs. This result indicated that the modified MWCNTs prepared in the study improved the waterproof performance, impact resistance, and flexibility of MWCNTs. This result suggested that modified MWCNTs enhanced the bonding force between the CC and the sheet by introducing F organic long-chain molecules. This long-chain molecule allowed the interaction between the coating and the sheet to change from the original weak van der Waals forces to stronger chemical bonds, which significantly improved the adhesion strength and impact strength between the coating and the sheet. The results were similar to those obtained by Wu et al. in a related study in 2022 [22]. The study was carried out to test the CR of CCs based on modified MWCNTs. The CCs were immersed in 3.5 wt% NaCl-S for 8 days, and the resistance values were found to be much higher than 109 Ω·cm2. The resistance values of all the CCs were in a stable state with slow fluctuation. The results indicated that the addition of modified MWCNTs made an additional layer of impedance arc on the surface of the coating, so that the corrosive medium was blocked out. In addition, the study also tested the prepared CCM based on modified MWCNTs for SHA. It was found that the Rct value of the coating was 1.107 × 104 Ω·cm2 at 6 h of immersion in 3.5 wt% NaCl-S, and the Rct of the coating was 1.025 × 106 at 30 days. The results demonstrated that the CC based on modified MWCNTs had excellent SHA and anti-corrosion ability.
5 Conclusion
Aiming at the poor anticorrosive and waterproof performance of the current AR coating materials for marine vessels, the study proposed a kind of modified MWCNTs by accessing FAS and SiO2 into the MWCNTs. Moreover, the AR was modified, and the CCM based on the modified MWCNTs was prepared with a view that the composite material could improve the anticorrosive and waterproof performance of traditional AR coating materials for marine vessels. The study first introduced the preparation methods of modified MWCNTs and modified AR, after which the properties of the prepared modified MWCNTs and CCM based on modified MWCNTs were examined. The modified MWCNTs were subjected to FAS and SiO2 access tests, water resistance, and anti-corrosion tests. The results showed that FAS and SiO2 were successfully incorporated into MWCNTs, and the surface of MWCNTs was covered by FAS and SiO2 to form a thin film, which could prevent the intrusion of water and corrosive media. The CCM based on modified MWCNTs was tested. The outcomes revealed that the CCM had good impact resistance, CR, water resistance, and SHA. One of the study's limitations is that neither the CC nor the modified MWCNTs' resistance to salt was investigated. Furthermore, the performance test was not sufficiently robust. Consequently, future research should examine the performance of the coating in different environments through a salt resistance experiment.
Funding
The research is supported by General Project of Basic Science (Natural Science) Research in Higher Education Institutions in Jiangsu Province: Construction of High Temperature Formic Acid Corrosion Resistant Coating on Cast Iron HT250 (21KJB580010); Jiangsu Maritime Vocational and Technical College Science and Technology Innovation Fund Project: Construction and Performance Research of High Temperature and Corrosion Resistant Coating on Cast Iron Pistons for Marine Methanol Fuel Engines (No.: 2021kjcx004).
Conflicts of interest
The authors declare that there’s no conflict of interest.
Data availability statement
All data generated or analyzed during this study are included in this article.
Author contribution statement
The nanocomposites based on modified multi-walled carbon nanotubes proposed in the study can improve the anticorrosive properties of acrylic resin coating materials. J. H. analyzed the data and C. S. helped with the constructive discussion. J. H. and C. S. made great contributions to manuscript preparation. All authors read and approved the final manuscript.
References
- Z. Liu, X. Zheng, H.H. Zhang, W. Li, R. Jiang, Review on formation of biofouling in the marine environment and functionalization of new marine antifouling coatings, J. Mater. Sci. 57 (2022) 18221–18242 [Google Scholar]
- D. Thomas, E. Philip, R. Sindhu, S.B. Ulaeto, Pugazhendhi, Developments in smart organic coatings for anticorrosion applications: a review, Biomass Convers. Biorefin. 12 (2022) 4683–4699 [Google Scholar]
- K. Ramachandran, V. Boopalan, J.C. Bear, R. Subramani, Multi-walled carbon nanotubes (MWCNTs)-reinforced ceramic nanocomposites for aerospace applications: a review, J. Mater. Sci. 57 (2022) 3923–3953 [Google Scholar]
- D. Prokić, M. Vukčević, A. Mitrović, M. Maletić, A. Kalijadis, Janković-Častvan, Adsorption of estrone, 17β-estradiol, and 17α-ethinylestradiol from water onto modified multi-walled carbon nanotubes, carbon cryogel, and carbonized hydrothermal carbon, Environ. Sci. Pollut. Res. 29 (2022) 4431–4445 [Google Scholar]
- M. Sabet, Multi-walled carbon nanotube reinforcement in polypropylene nanocomposites: comprehensive analysis of thermal behavior, mechanical properties, and dispersion characteristics, J. Thermal Anal. Calorm. 149 (2024) 3165–3179 [Google Scholar]
- J. Kumar, K. Kumar, B. Jaiswal, K. Kumar, R.K. Verma, Investigation on the physio-mechanical properties of carpet waste polymer composites incorporated with multi-wall carbon nanotube (MWCNT), J. Text. Inst. 114 (2023) 664–673 [Google Scholar]
- Z. Jia, M. Kong, B. Yu, Y. Ma, J. Pan, G. Wu, Tunable Co/ZnO/C@ MWCNTs based on carbon nanotube-coated MOF with excellent microwave absorption properties, J. Mater. Sci&. Technol. 127 (2022) 153–163 [Google Scholar]
- M. Rezaei Abadchi, M. Mirzaee, E. Dorkhani, A. Zolriasatein, N.R. Noori, Surface modification of acrylic coating with anti‐corrosion and anti‐UV materials, J. Chin. Chem. Soc. 69 (2022) 912–924 [Google Scholar]
- W. Jiang, X. Wen, Y. Jiang, H. Lu, T. Zhou, Novel anticorrosive coating of silicone acrylic resin modified by graphene oxide and polyaniline, Corros. Rev. 40 (2022) 501–511 [Google Scholar]
- S.M. Abdulkareem, R.M. Alsaffar, G.H.A. Razzaq, J.H. Mohammed, T.M. Awad, Effect of direct and indirect in-situ sonochemical synthesis methods of MWCNTs–CoNiFerrite on the hydrogen storage, J. Sol-Gel Sci. Technol. 111 (2024) 979–988 [Google Scholar]
- J. Zhao, S. Tian, H. Xie, X. Zhang, Study of time-varying laws of stability and wettability of SiO2–H2O nanofluids with different particle sizes, Ind&. Eng. Chem. Res. 62 (2023) 13529–13540 [Google Scholar]
- S. Huang, R.G. Fedorov, Y. Ein-Eli, Silicon-coated multi-walled carbon nanotube (MWCNT) tissues as flexible free-standing anodes for advanced Li-ion batteries, J. Solid State Electrochem. 28 (2024) 2139–2149 [Google Scholar]
- Q. Xie, G. Yin, Q. Duan, Y. Zhong, J. Xie, K. Fu, The fluorinated SiO2 self‐assembly surface of epoxy resin with excellent insulating and superhydrophobic properties, Polym. Compos. 44 (2023) 6071–6082 [Google Scholar]
- J. Zoubir, Y. Elkhotfi, S. Qourzal, M. Tamimi, A. Tounsi, A. Assabbane, One-step electrodeposition of silver nanoparticles on multi-walled carbon nanotubes to fabricate an Ag-NPs@ MWNTC&CPE sensor for the detection of the toxic antibiotic ronidazole in doped chicken liver and muscle, Anal. Chem. Lett. 13 (2023) 539–560 [Google Scholar]
- H.Z. Ma, J.N. Zhao, R. Tang, Y. Shao, K. Ke, K. Zhang, Polypyrrole@ CNT@ PU conductive sponge-based triboelectric nanogenerators for human motion monitoring and self-powered ammonia sensing, ACS Appl. Mater&. Interfaces 15 (2023) 54986–54995 [Google Scholar]
- J. Zoubir, Y. Elkhotfi, S. Qourzal, M. Tamimi, A. Tounsi, A. Assabbane, One-step electrodeposition of silver nanoparticles on multi-walled carbon nanotubes to fabricate an Ag-NPs@ MWNTC&CPE sensor for the detection of the toxic antibiotic ronidazole in doped chicken liver and muscle, Anal. Chem. Lett. 13 (2023) 539–560 [Google Scholar]
- S. Kumar, H.K. Sidhu, A.K. Paul, N. Bhardwaj, N.S. Thakur, A. Deep, Bioengineered multi-walled carbon nanotube (MWCNT) based biosensors and applications thereof, Sens. & Diagn. 2 (2023) 1390–1413 [Google Scholar]
- M. Asaftei, M. Lucidi, C. Cirtoaje, A.M. Holban, C.A. Charitidis, Fighting bacterial pathogens with carbon nanotubes: focused review of recent progress, Rsc Adv. 13 (2023) 19682–19694 [Google Scholar]
- A. Zhou, H. Yu, J. Tang, B. Zhang, F. Qi, Y. Zhou, N-PMI modified PAZ nanocomposite coatings with self-healing function for anticorrosion and antifouling applications, Prog. Org. Coat. 180 (2023) 107589 [Google Scholar]
- T. Liu, Z. Zhou, L. Zhang, W. Zhang, W. Yang, Novel electrochemical sensor based on molecularly imprinted polymers with MWCNTs-SiO2 for selective and sensitive detecting 2, 4-D, J. Inorg. Organomet. Polym. Mater. 32 (2022) 572–582 [Google Scholar]
- Y. Meng, J. Cheng, C. Zhou, Superhydrophobic and stretchable carbon nanotube/thermoplastic urethane-based strain sensor for human motion detection, ACS Appl. Nano Mater. 6 (2023) 5871–5878 [Google Scholar]
- Y. Wu, J. Du, G. Liu, D. Ma, F. Jia, J.J. Klemeš, A review of self-cleaning technology to reduce dust and ice accumulation in photovoltaic power generation using superhydrophobic coating, Renew. Energy 185 (2022) 1034–1061. [Google Scholar]
Cite this article as: Jie Hui, Changfei Sun, Preparation and performance of modified MWCNTS nanocomposites for ship anti corrosion, Manufacturing Rev. 12, 17 (2025), https://doi.org/10.1051/mfreview/2025012
All Tables
All Figures
 |
Fig. 1 Schematic diagram of MWCTNS. |
| In the text | |
 |
Fig. 2 Preparation method of modified MWCNTs. |
| In the text | |
 |
Fig. 3 Preparation process of modified AR. |
| In the text | |
 |
Fig. 4 Preparation process of CC materials for modified MWCNTs. |
| In the text | |
 |
Fig. 5 X-ray diffraction analysis and infrared spectrum analysis results of MWCNTs-OH and MWCNTs-OH@SiO2. |
| In the text | |
 |
Fig. 6 X-ray photoelectron spectroscopy of modified MWCNTs. |
| In the text | |
 |
Fig. 7 Micromorphologies of MWCNTs, MWCNTs-OH@SiO2, and FAS-MWCNTs-OH@SiO2. |
| In the text | |
 |
Fig. 8 Results of contact angle test, adhesion test, impact resistance test, and flexibility test. |
| In the text | |
 |
Fig. 9 Nyquist curve and Bode map results of CC immersed in 3 wt%NaCl-S for 8 days. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.